Security
Latest about Security
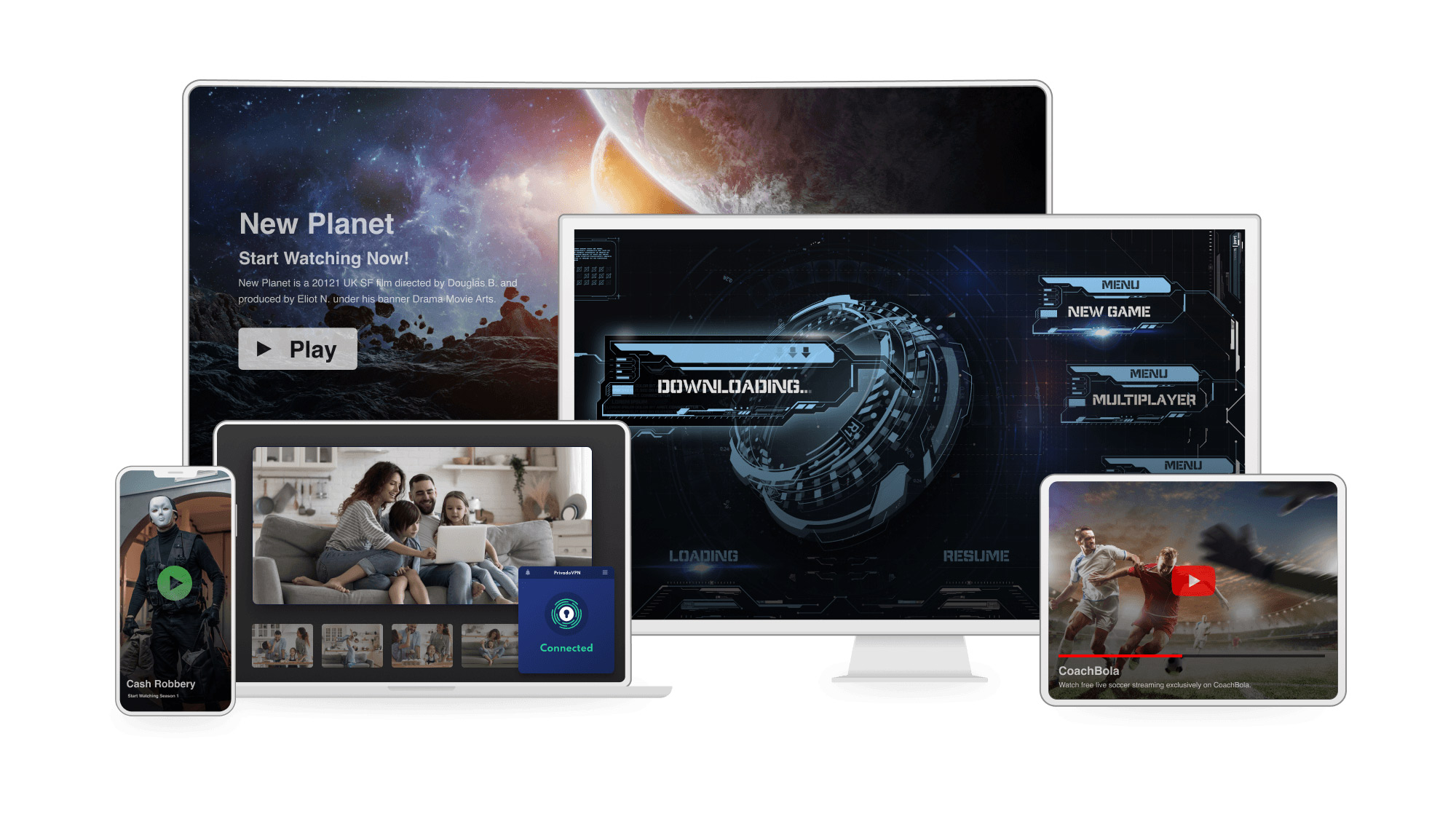
PrivadoVPN Free review
By Kristin Hassel last updated
PrivadoVPN Free gives you more servers to choose from than most free VPNs, but you may be a bit disappointed that not all of its advanced features are available for every OS.

Windscribe VPN Free review
By Michael Simon last updated
Windscribe’s free plan is one of the best on the market, and it’s a strong option for streaming, torrenting, and private browsing, but it still has some areas for improvement

Over 40,000 security camera feeds found exposed online – here’s how to protect yours
By Amber Bouman published
Unsecured feeds from homes and businesses were streaming live on the internet
The best torrenting VPN in 2025
By Mo Harber-Lamond last updated
VPN If you're sharing P2P, it's absolutely essential you protect yourself with a torrenting VPN. Here are the five best VPNs for torrenting today.

The best China VPN: working services right now
By Mo Harber-Lamond last updated
VPN A China VPN is essential for anyone traveling to the country – but not every provider works. Here are the very best VPNs for China on the market.

The best BBC iPlayer VPN
By Olivia Powell last updated
VPN From catching up on sporting events, to Doctor Who and Strictly Come Dancing, the BBC has it all. A BBC iPlayer VPN is your ticket to entry

The best Iran VPN
By Krishi Chowdhary last updated
VPN Avoid widespread censorship facing citizens and travelers in Iran by using our top 5 Iran VPN choices.

16 billion password data breach hits Apple, Google, Facebook and more — LIVE updates and how to stay safe
By Darragh Murphy last updated
The latest updates on the one of the largest data breaches in history, how to find out if you're affected and how to stay safe.
Here at Tom’s Guide our expert editors are committed to bringing you the best news, reviews and guides to help you stay informed and ahead of the curve!


